Chemical Bonding
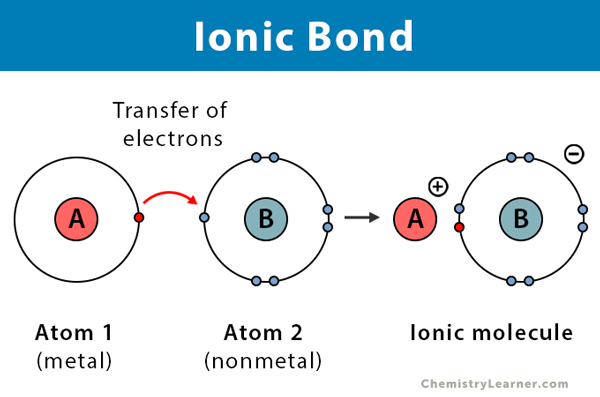
Ionic Bonding
- Metal + Non-Metal
- Metal gives electron(s) to Non-Metal
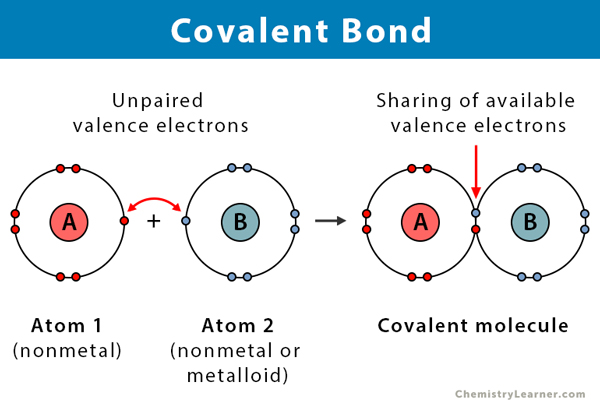
Covalent Bonding
- Non-Metal + Non-Metal
- Shares electron(s)
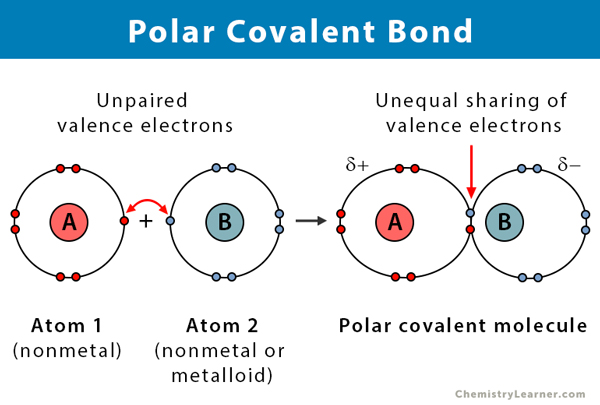
Polar Covalent Bonds
- Non-Metal + Non-Metal
- Shares electron(s)
- Un-equal sharing of the electrons due to Electronegativity
- Difference in Electronegativity must be greater than 0.5 to be considered polar
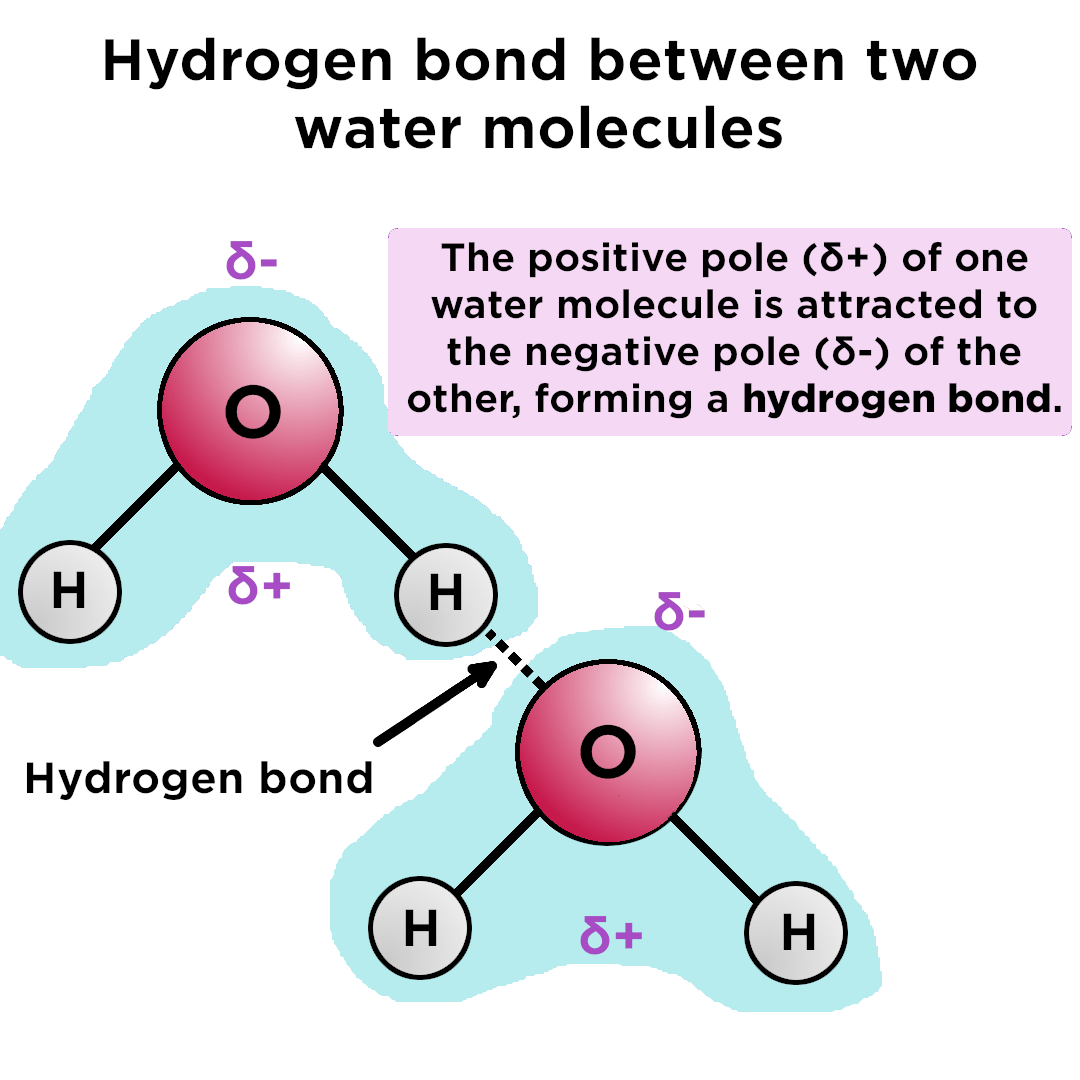
Hydrogen Bonding
- Intermolecular Bonds
- Occur between Polar Molecules
- An electromagnetic attraction between two separate molecules, or within the same molecule
- Not a true bond
Ionic Bonding Example
Covalent Bonding Example
Polar Covalent Bonding Example
Hydrogen Bonding Example